Departments
- Home
- Departments
How it works
Departments

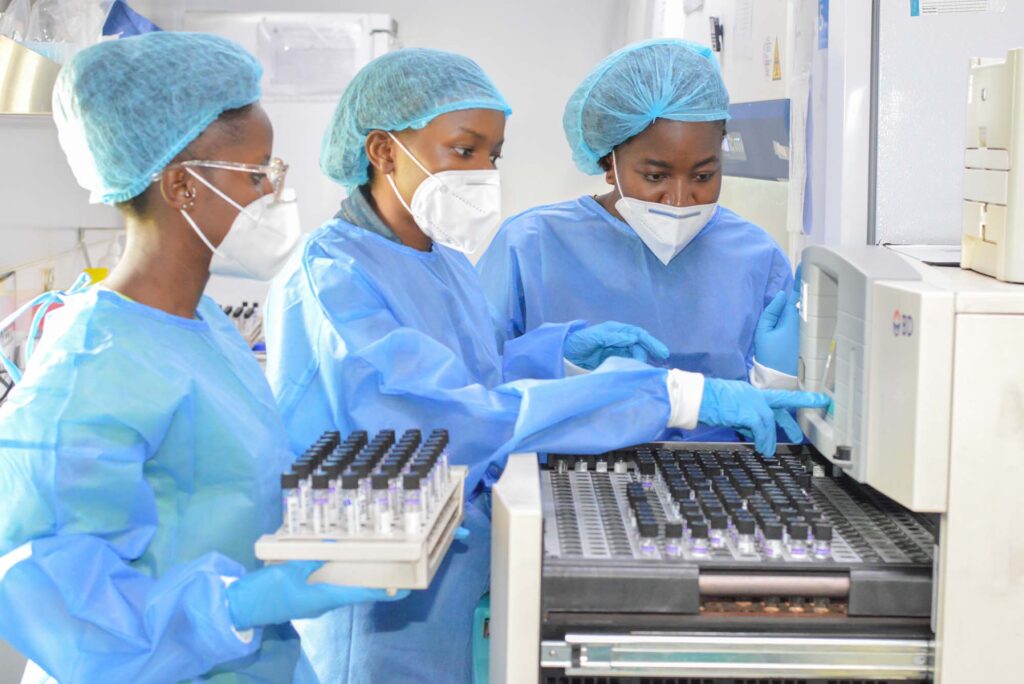
DSM
This technical section is the genesis of TB diagnostic services offered at NTBRL. The section is responsible for the following functions:
- Sample reception, registration and initial processing.
- All Presumptive cases are tested using Genexpert MTB RIF/Ultra assay
- ZN microscopy for follow up cases
- Covid-19 testing (PCR)

Culture & Identification
In this section samples are received from DSM and processed for TB Culture. TB culture includes specialized liquid culture (MGIT) and the solid culture (L.J). Mycobacterium species are generally slow growers, hence processing culture samples and release of results takes a maximum of 9 weeks.

Phenotypic Drug Susceptibility Testing (DST)
Culture positive samples are processed for first and second line DST. DST is performed using the specialized liquid culture technology (MGIT). DST results take a maximum of 21 days from the time of culture positivity.

Genotypic Drug Susceptibility Testing (LPA)
Molecular technology for the detection of genetic mutations associated with resistance to anti TB drugs. A specialized technique performed for first and second line drugs.

STORES
Responsible for inventory management, distribution and supply chain. Functions as a repository of reagents, consumables, stationery and equipment.

ADMIN
Responsible for the general day to day activities including but not limited to: Clientele reception, communications, scheduling and overall administration of operations.

House Keeping
Maintains the general outlook of the laboratory, from inside to outside.

Data
Responsible for making sense of TB data. The section provides information to clients, management and release of results coupled with production and maintenance of data tools which help monitor Drug resistant & Drug sensitive TB patients.

Quality Desk
The law enforcers in the context of quality management systems at NTBRL. This important section has the mandate to ensure all personnel at NTBRL perform their duties in real time and conforming to ISO15189:2012 requirements. Additionally, the section is responsible for analysis and reporting on the implementation, outcomes, areas of improvement and recommendations from the quality management systems.
Proficiency testing
The Proficiency Testing Unit, within the framework of quality management systems at NTBRL, plays a crucial role in ensuring the accuracy and reliability of laboratory testing related to public health concerns. This section is mandated to coordinate and monitor all proficiency testing activities in accordance with ISO 15189:2012 standards. Furthermore, it oversees the evaluation and reporting of performance outcomes, identifies areas requiring improvement, and provides recommendations to enhance the overall quality and consistency of laboratory testing processes.

